In assessing the obviousness of chemical species when prior art teaches an encompassing genus, Section 2144.08 of the MPEP advises us to consider any teaching or suggestion in the reference of a preferred species or subgenus that is significantly different in structure from the claimed species or subgenus—because such a teaching may weigh against selecting the claimed species or subgenus. See M.P.E.P. § 2144.08, para. II.A.4(c).
For example, as explained in the Manual, “teachings of preferred species of a complex nature within a disclosed genus may motivate an artisan of ordinary skill to make similar complex species and thus teach away from making simple species within the genus.” Id. (citing In re Baird, 15 F.3d 380, 29 U.S.P.Q.2d 1550 (Fed. Cir. 1994)) (emphasis added).
A recent opinion of the Patent Trial and Appeal Board (“Board”) illustrates a scenario where the disclosure of preferred compounds having a complex chemical structure fails to provide motivation under Section 103 to select simpler compounds encompassed by a broad chemical genus.
On July 14, 2020, the Board issued a decision in Ex parte Biediger reversing obviousness rejections premised on structural similarity of chemical compounds falling with the scope of a broad genus, where the prior art reference disclosed a preference for species having a relatively complex chemical structure.
Claim 1 of the subject application reads as follows:
1. A compound of formula I having a chemical structure of
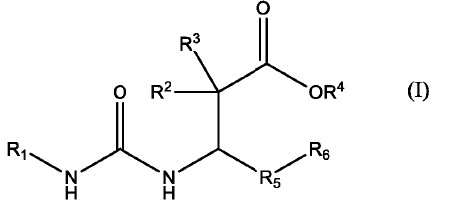
wherein R1 is
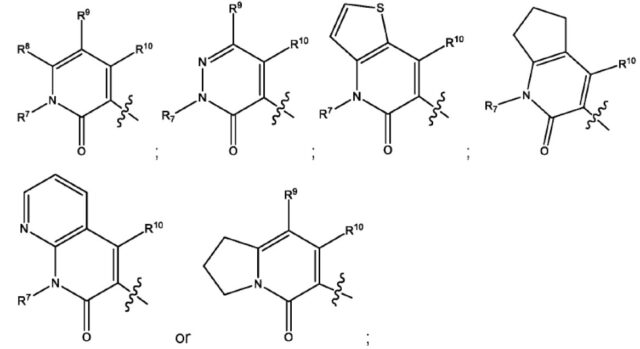
R2 and R3 are independently hydrogen or C1-4 alkyl;
R4 is H or C1-4 alkyl;
R5 is phenyl, or aryl, any of which is substituted with one or more of hydrogen, C1-4 alkyl, alkoxy, oxo, halogen, haloalkoxy, -CF3, hydroxyl, -OCF3, aryl, -OCF2H, -OCF2 CF2H, -O(C3-6 cycloalkyl), -OCH2CF3, thioalkoxy, dialkylamino, C3-6 cycloalkyl or haloalkyl;
R6 is phenyl, or aryl, any of which is substituted with one or more of hydrogen, C1-4 alkyl, alkoxy, halogen, oxo, acetyl, haloalkoxy, -CF3, hydroxyl, -OCF3, aryl, -OCF2H, -OCF2CF2H, -O(C3-6 cycloalkyl), -OCH2CF3, thioalkoxy, dialkylamino, C3-6 cycloalkyl or haloalkyl;
R7 is H or C1-4 alkyl;
R8 and R9 are independently hydrogen, C1-4 alkyl, or hydroxyl;
R10 is hydroxyl;
or a pharmaceutically acceptable salt or stereoisomer thereof.
Dependent Claim 8 of the subject application further limits the R1 group to a sub-genus of compounds including a compound provisionally elected by the applicant in response to a restriction requirement. Claim 8 reads as follows:
8. The compound of claim 1, wherein the compound offormula I is the compound of formula IA having a chemicalstructure of
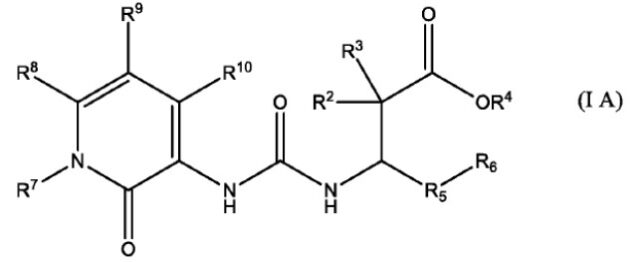
wherein, R2, R3, R4, R5, R6, R7, R8, R9 and R10 are as defined in claim 1; or a pharmaceutically acceptable salt or stereoisomers thereof.
The Examiner rejected the claims of the subject application as being obvious over US 6,972,296 (“Biediger”), which discloses a large genus of compounds encompassing the compound elected by the Applicant.
The obviousness rejections relied upon the genus of Formula V below – compounds of which are described as being “preferred compounds” in Biediger. The Examiner found that the R18 group of Formula V (corresponding to the R7 group in Claims 1 and 8), and the other groups in Formula V, were defined in a manner that encompasses the Applicant’s elected species.
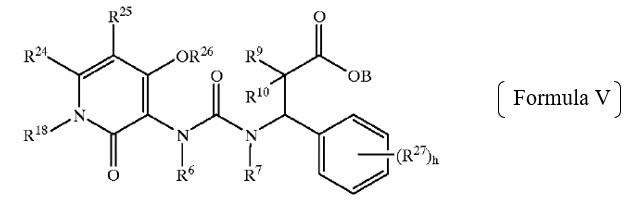
While acknowledging that Biediger does not specifically disclose any compounds reading on the claimed genus, the Examiner found that (a) Biediger teaches that the compounds of Formula V have the same utility (i.e., as integrin inhibitors) as the claimed compounds, and that (b) persons of ordinary skill in the relevant art would have recognized the close structural similarity and would have expected the respective compounds to have similar properties.
Relying in part on Section 2144.08 of the Manual, the Applicant persuaded the Board to reverse the obviousness rejections over Biediger, because the preferred compounds of Formula V that are actually disclosed in this reference included relatively complex benzylic R18 groups compared to the simpler “H or C1-4 alkyl” groups of R7 in Claims 1 and 8.
As explained below, the Board agreed that disclosure of the preferred compounds having “benzylic or bulkier” groups as the R18 group of formula V, in the experimental section of Biediger, expressed a preference for more complex substituents relative to the simpler “H or C1-4 alkyl” groups of R7 in the subject application.
Based on the current record, we determine that Appellant has the better argument. The generic chemical structure in Formula V of Biediger has ten variable groups, each of which is “independently selected” from large groups of widely–variable chemical substituents. See Biediger, 7:44–8:44. For example, R6, R7, and R18 are described as being “independently selected from the group consisting of alkyl, alkenyl, alkynyl, hydroxyalkyl, aliphatic acyl, alkynylamino, alkoxycarbonyl, heterocycloyl, -CH=NOH, haloalkyl, alkoxyalkoxy, carboxaldehyde, carboxamide, cycloalkyl, cycloalkenyl, cycloalkynyl, cycloalkylalkyl, aryl, aroyl, aryloxy, arylamino, biaryl, thioaryl, diarylamino, heterocyclyl, alkylaryl, aralkenyl, aralkyl, alkylheterocyclyl, heterocyclylalkyl, carbamate, aryloxyalkyl, hydrogen and –C(O)NH(benzyl) groups.” Id. at 8:16–25. Given the number, breadth, and possible permutations of these variable groups, we agree with Appellant that the genus taught in Biediger is “extremely large.” Appeal Br. 6.
We are also persuaded by Appellant’s argument that Biediger expresses a preference for more complex substituents at the R18 position in Formula V. See Appeal Br. 6–9. Biediger teaches that “[p]resently preferred compounds of Formula V” have a “substituted or unsubstituted aralkyl” at R18. Biediger, 8:45–47. In addition, Biediger describes the synthesis of a multitude of compounds. See id. at 26:1–53:10 (describing synthetic schemes); Table 1 (listing H1 NMR spectra for synthesized compounds). Appellant “submits that all of the[se] compounds . . . have bulky groups (unsubstituted aralkyl or substituted aralkyl) at [the] R18 position.” Appeal Br. 9; see also id. at 10 (“[T]he compounds synthesized in Biediger are preferred species.”). Examiner does not dispute that Appellant’s characterization is accurate. Accordingly, we agree with Appellant that Biediger expresses a clear preference for R18 substituents other than the hydrogen or relatively short alkyl group recited in Appellant’s claims.
Appeal No. 2019-006528, at 6-7 (P.T.A.B. July 14, 2020) (non-precedential) (emphasis added).
The Board also concluded that the Examiner’s findings concerning “shared utility” and the “level of skill in the art” were not sufficient to support the obviousness rejection. Although the Board acknowledged that such considerations are relevant to the Section 103 analysis, the Board concluded that the Examiner in this case had not sufficiently explained why motivation would have existed to use “relatively simple substituents” at the R18 position in formula V of Biediger.
Examiner’s other findings concerning shared utility and the level of skill in the art (see Final Act. 5–6) are insufficient to support a rejection on the record here. Teachings of similar uses and the relative level of skill in the art are relevant considerations in assessing whether a species or subgenus would have been obvious over the disclosure of a genus in the prior art. See MPEP § 2144.08. However, the MPEP makes clear that the rejection must also “consider the size of the genus” and “any teaching or suggestion in the reference of a preferred species or subgenus that is significantly different in structure from the claimed species or subgenus.” Id. In this case, Examiner has not sufficiently articulated why one of ordinary skill in the art would have been motivated to use the relatively simple substituents in Appellant’s claims at the R18 position given Biediger’s clear preference for more complex groups at that position, particularly given the enormous breadth of the genus encompassed by the generic structure in Formula V.
Id. at 7 (emphasis added).
Takeaway: In assessing obviousness rejections of chemical compositions, it is important to fully consider the teachings of prior art references as a whole – especially when the Patent Office is relying upon a chemical genus encompassing a large breadth of compounds. When preferred compounds of relatively complex structure are disclosed in the prior art, one should carefully assess whether such disclosure would discourage the selection of simpler compounds falling within the genus.
Judges: E.B. GRIMES, E.A. LaVIER and M.A. VALEK (Opinion by VALEK)
