Ex parte Fox is a recent decision of the Patent Trial and Appeal Board (PTAB) addressing the interpretation of a Markush group in a claim rejected for anticipation and obviousness.
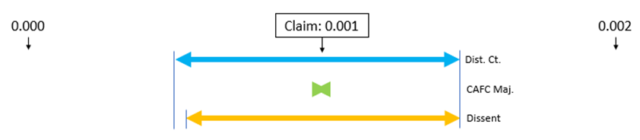
The claim at issue was directed to “[a] cleaning solution comprising one or more solvents selected from the group consisting of acetoacetates, alcohols, glycol ethers, glycol esters, terpenes, and water…” The claim also required other components and properties.
The examiner identified a cleaning solution in the prior art which included some of the solvents listed in the claim. However, the prior art cleaning solution required a further solvent, “at least one oxyisobutyric acid ester.” The examiner asserted that, although at least one of the listed solvents must be present, the “comprising” language in the claim permitted the presence of additional solvents in the cleaning solution.
The examiner supported his position by citing MPEP 2111.03.II, which, in turn, cites In re Crish, 393 F.3d 1253 (Fed. Cir. 2004). The examiner relied on the following description of the Crish case from the MPEP:
In determining the scope of applicant’s claims directed to “a purified oligonucleotide comprising at least a portion of the nucleotide sequence of SEQ ID NO:1 wherein said portion consists of the nucleotide sequence from [521] to 2473 of SEQ ID NO:1…” the court stated that the use of “consists” in the body of the claims did not limit the open-ended “comprising” language in the claims…
The PTAB stated that a case cited in the same paragraph of the MPEP relied on by the examiner, Multilayer Stretch Cling Film Holdings, Inc. v. Berry Plastics Corp., 831 F.3d 1350, (Fed. Cir. 2016), included a fact pattern much closer to the claim on appeal. The PTAB stated that Crish was distinguishable from the claims on appeal, because Crish did not involve a Markush group of distinct alternatives from which to select.
Relying on the Multilayer case, the PTAB noted that the use of the transitional phrase “consisting of” to set off a patent claim element creates a very strong presumption that that claim element is closed and therefore excludes any elements, steps, or ingredients not specified in the claim. Thus, if a patent claim recites “a member selected from the group consisting of A, B, and C,” the member is presumed to be closed to alternative ingredients D, E, and F. In the claim on appeal, the “member” was the “solvent,” so the claim was closed to solvents other than those specifically recited in the Markush group – even though the claimed cleaning solution was open to containing other non-solvent elements.
When the claim on appeal was properly construed, the prior art was not sufficient to anticipate or render obvious the claim, because the prior art required the presence of “at least one oxyisobutyric acid ester” solvent, i.e, a solvent that was not recited in the Markush group of the claim.
Takeaway: Markush groups are frequently used to define components of composition. It is important to be intentional when drafting Markush group to control what is within and outside the scope of the claim. In thinking about a claim drafted in typical Markush format, “an X selected from the group consisting of A, B, and C,” attention is often focused on A, B, and C, which define what must be present. However, care must be taken in defining X which indicates what will be excluded from the claim. A broad X may define over the prior art but may also make design-around compositions easy to identify.
Judges: Cashion, McGee, Inglese



 .
.
 .
.