Recently we reviewed the USPTO’s published Examiner guidance concerning “the flexible approach to determining obviousness that is required by KSR Int’l Co. v. Teleflex Inc. (KSR)” in which the Office instructed Examiners to avoid any type of formalistic or formulaic approach when providing a reason to modify the prior art in favor of relying on any possible source that “may, either implicitly or explicitly, provide reasons to combine or modify the prior art to determine that a claimed invention would have been obvious.” Unfortunately for applicants, this advice seems to have accurately captured the current sensibility of the Federal Circuit which, in Janssen Pharms., Inc. v. Teva Pharms. USA, Inc., No. 2022-1258 (Fed. Cir. Apr. 1, 2024), recently openly admonished a District Court for using a “degree of rigidity” in its obviousness analysis that was foreclosed by KSR and, perhaps most devastatingly, for “ask[ing] the wrong questions about important aspects of the obviousness inquiry.”
In particular, and among the district court’s several identified errors, the Federal Circuit in Janssen found that the district court analyzed the prior art without giving the needed weight to the perspective of a person of ordinary skill in the art (POSA) who is “capable of deducing what references fairly suggest or employing ordinary creativity.” And instead of considering the prior art in context or in combination, the Federal Circuit criticized the district court’s consideration of each reference one-by-one, “identifying each difference or dissimilarity between an individual reference and the claims, but not fully assessing the teachings in toto” thereby yielding a “siloed and inflexible approach” that left insufficient room for consideration of how background knowledge in the art would have impacted a POSA’s understanding of, or motivation to modify, the primary references at issue.” This approach, in the view of the Federal Circuit, inflated the significance of “minor variations between the prior art and the claims,” leading to the district court’s erroneous conclusion of nonobviousness.
Given the general decorum typically surrounding court opinions, this level of direct and unfettered criticism can mean only one thing – that at least for the foreseeable future the USPTO guidelines got it right insofar as the Federal Circuit is concerned. As the Federal Circuit, citing KSR, emphasized in this case, “[a]ssessing obviousness is based on an ‘expansive and flexible approach’ that ‘need not seek out precise teachings directed to the specific subject matter of the challenged claim, for a court can take account of the inferences and creative steps that a person of ordinary skill in the art would employ’.” Applicant beware.




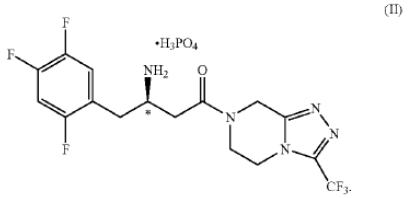 .
.





